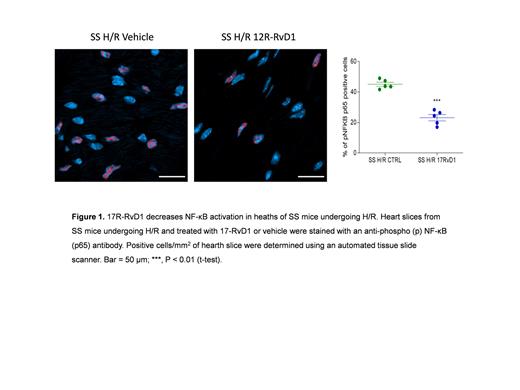
Sickle cell disease (SCD) is a severely invalidating genetic red cell disorder. Inflammatory vasculopathy and cardiopulmonary complications markedly impact quality of life and survival. Limited studies are available on the pathogenesis of sickle cell related cardiomyopathy. However, the combination of inflammatory vasculopathy, heart ischemia, and myocardial fibrosis have been suggested to play a role in sickle cell related cardiomyopathy. This is supported by the amelioration of cardiomyopathy in SCD patients, early and intensively treated with either hydroxyurea or chronic transfusion regimen (Niss O et al. Blood 140: 1322, 2022). Recently, we have highlighted an impairment of pro-resolving events in SCD with a deleterious effect on acute sickle cell related organ damage (Matte A et al. Blood 133: 252, 2019). Growing evidence links impairment of pro-resolving mechanisms with atherosclerosis and ischemic cardiomyopathy (Kain V et al. J Mol Cell Cardiol 84: 24, 2015; Conte MS et al. JCI 128: 3727, 2018). Among specialized pro-resolving mediators, 17R-resolvin D1 (17R-RvD1) has been shown to play important protective actions in cardiovascular diseases (Kain V et al. J Mol Cell Cardiol 84: 24, 2015). Here, we first determined the appropriate window of time to study cardiac response to hypoxia/reoxygenation (H/R) stress, mimicking sickle cell related acute vaso-occlusive crisis. We show that humanized SCD mice (TT model) exposed to H/R (10 hours hypoxia 8% oxygen, followed by 3 hours reoxygenation) display increased markers of myocardial dysfunction with neutrophil heart infiltration compared to either SCD mice under normoxia or to healthy (AA) animals exposed to H/R stress. With integrated proteomic and miRNA analyses we have identified upregulation of proteins and miRNAs involved in fibrosis, hypertrophy of the heart, and cardiomyocyte apoptosis. Differentially expressed miRNAs in SCD-hearts compared to AA included miR-16-5p, miR-122-5p, and miR-206-3p, which are involved in angiogenesis and heart fibrosis and hypertrophy. Upregulated proteins in SCD hearts in response to H/R encompassed: NF-kB, Nrf2, hemeoxygenase-1, glutathione peroxidase, and peroxiredoxins (associated to response to oxidative stress), the profibrotic factors FGF and PDGF as well as the TGF-ß1 receptor, and the pro-angiogenic VEGF pathway. Treatment with 17R-RvD1 (100 ng administrated by gavage 1 hour before H/R, see also Matte A et al. Blood 133: 252, 2019) protected heart of SCD mice from H/R-induced stress as supported by significant reduction of ANP, a marker of cardiovascular dysfunction, decrease in neutrophil infiltration, and NF-kB activation (Figure 1). 17R-RvD1 also diminished the H/R-driven upregulation of miR-16-5p, while it enhanced miR-206-3p that was downregulated by H/R, suggesting that 17R-RvD1 can reduce cardiac fibrosis and other pathological processes associated with these miRNAs in SCD. We also found that 17R-RvD1 administration to SCD mice blunted the activation of TGF-ß1 pathway and of markers of extracellular remodeling, supporting the beneficial effect of 17R-RvD1 against H/R stress. Taken together, our data show that 17R-RvD1 reduced heart remodeling and fibrosis in SCD mice undergoing H/R, a model of acute VOCs. This improved understanding in basic pathophysiology of SCD-related inflammatory cardiomyopathy, providing new evidence for protective therapeutic effects of 17R-RvD1 on SCD cardiovascular diseases.
Disclosures
Brugnara:Sysmex: Honoraria; Pfizer: Honoraria; Garuda Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria. De Franceschi:Bristol Myers Squibb: Research Funding; Agios: Research Funding; F. Hoffmann-La Roche Ltd, Basel: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal